According to a telephonic conference held on May 10, 2019, a settlement has been reached for a Bard IVC Filter lawsuit before the trial date; however, the settlement agreement details remain confidential.
Full Answer
What happened to the Bard IVC filter lawsuit?
In May 2019, lawyers announced a settlement had been reached in the mass litigation over Bard IVC filters in an Arizona federal court. The litigation was closed to new cases and anyone who filed a lawsuit to be part of the litigation after May 31, 2019, would be given a full refund of the filing fee.
What is the settlement for IVC filter lawsuit?
IVC filter lawsuits claim C.R. Bard and Cook Medical’s devices were defective, making them more likely to fracture or perforate the inferior vena cava. In March 2018, Bard was ordered to pay a woman $3.6 million to settle an IVC filter case.
What are some of the most recent IVC filter verdicts?
The most recent was a $3.3 million verdict in Wisconsin for a woman with a Bard Meridian IVC Filter. Her IVC filter became embedded in her vein. She required several surgeries to both remove the filter and to collect the broken pieces of the shattered filter. In July, a jury awarded $386,250 in a fractured filter case against C.R. Bard.
What is the MDL for Bard IVC filters?
This MDL involved many Bard filters: the G2, G2X, the Recovery, Meridian, Eclipse, and Denali. Cook MDL: Southern District of Indiana. If you or a loved one has suffered serious side effects and deaths caused by IVC filters, call an IVC filter lawyer today at 800-553-8082 or get a free, no-obligation online case review of your legal claim.

How much compensation do you get for an IVC filter?
Based on the IVC filter verdicts and the history of mass tort litigation, settlements may average between $100,000 and $500,000 for significant injury cases but there will certainly be cases that settle higher and lower than that payout range.
What IVC filters are recalled?
FDA Recalls of IVC Filters. The FDA has issued three Class I recalls of IVC filters – the Cordis OPTEASE filter, the Cordis OPTEASE filter introduction kit, and the Greenfield filter system. There are three FDA Class II recalls of IVC filters – the Bard Denali, the Greenfield, and the Cordis OPTEASE filters.
What happens when an IVC filter catches a clot?
When an IVC filter has captured a blood clot traveling through the inferior vena cava vein, the filter clogs and creates a host of medical symptoms, including: Swollen legs, Leg pain, and. The feeling of internal pressure in the legs.
How long should IVC filters stay in?
The U.S. Food and Drug Administration (FDA) recommends removing temporary IVC filters after 29-54 days. While this is not very long, it should provide enough time for the acute threat to pass or to find another solution that can work on a long-term basis.
What is the status of the IVC filter lawsuit?
The Ninth Circuit ruled in favor of the plaintiff in the first Bard IVC Filter bellwether trial, resulting in a $3.6 million verdict for a Georgia woman. The jury awarded $1.6 million in actual damages and $2 million in punitive damages to Sherr-Una Booker.
Should I have my IVC filter removed?
When should an IVC filter be removed? It is recommended that a removable filter be removed when the risk of a blood clot traveling to the lungs has passed, or if a patient can take blood thinners.
Can IVC filter cause back pain?
Abdominal or back pain associated with an IVC filter is typically due to penetration through the blood vessel wall, impingement against nearby nerves, and/or penetration into adjacent organs. Migration of filter components or referred pain may affect other areas of the body.
Do lungs heal after pulmonary embolism?
A pulmonary embolism (PE) is caused by a blood clot that gets stuck in an artery in your lungs. That blockage can damage your lungs and hurt other organs if they don't get enough oxygen. It's a serious condition, and recovery can take weeks or months.
Does drinking water help with blood clots?
Hydrate. Dehydration is thought to increase the odds of developing a blood clot. Therefore, it's important to drink plenty of water each day, especially if you have other risk factors for blood clots.
Can you leave a IVC filter in forever?
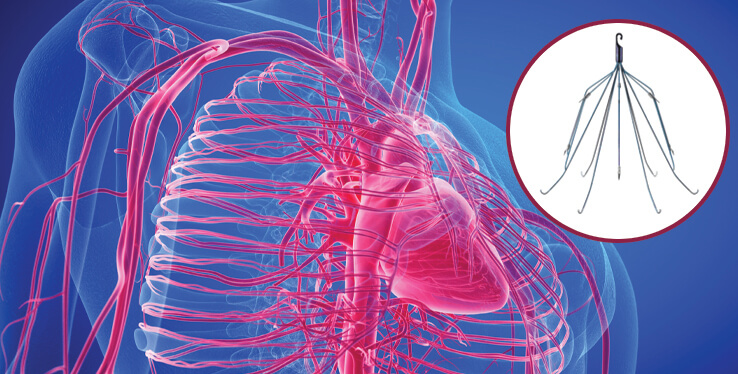
An IVC is a special basket-like filter to trap clots that can be inserted into the inferior vena cava, a large vein in the abdomen that carries blood from the lower to the upper half of the body to the heart. Doctors can implant an IVC filter permanently or temporarily, depending on the patient's needs.
Can IVC filter be removal after 10 years?
Embedded IVC Filters In many cases, IVC filters left in place too long, over several months to several years, cannot be safely removed using standard methods because they have scarred into the walls of the vein. “Unfortunately, this complication rate can be really high,” Dr.
Is there a permanent IVC filter?
In broad terms, modern IVC filters can be divided into two categories: permanent IVC filters (pIVCFs) and retrievable IVC filters (rIVCFs).
Are all IVC filters MRI safe?
IVC Filters a threat to MRI Safety. IVC filters can pose a threat to MRI safety, as some of the implantable filters are made of metal that can be attracted to the magnets used in MRIs. If precautions are not taken, people who have had an IVC filter implanted can suffer serious injuries if they have an MRI.
Are Greenfield filters safe?
They conclude that, while most Greenfield filters respond to a magnetic field, the chance of migration of a filter because of an MRI scan is small. Therefore, MRI scanning of patients with Greenfield filters has little risk.
Who makes Greenfield IVC filters?
Boston Scientific CorpTable 2NameManufacturerFDA approval year/MiscellaneousTitanium Greenfield IVC FilterBoston Scientific Corp Watertown, MA, USA1987Vena Tech LGM IVC FilterB Braun Evanston, IL, USA1989Cook Medical Birds Nest IVC FilterCook Incorporated Bloomington, IN, USA1989/indicated for inferior vena cava diameters of >28mm18 more rows•Jun 30, 2017
Who manufactures IVC filters?
1. Bard IVC filters. At this time, Bard is manufacturing the following IVC filters: DENALI® Vena Cava Filter, and.
Why Were IVC Lawsuits Unsuccessful at First?
For a long time, no vena cava filter lawsuits so far have made it through trial with a successful jury verdict. Yet many plaintiffs' attorneys rema...
How Much Compensation in an IVC Filter Settlement Can You Expect?
The settlement value of an IVC filter lawsuit is going to depend on the severity of the victim's injuries. If there is a global settlement, there w...
What IVC Filters are Recalled?
The FDA has issued a number of recalls and warnings on IVC filters. Two of the recalls were Class I recalls. Class I means there is a reasonable pr...
How Do You Join the IVC Filter Class Action?
The easiest path to join one of the MDL class actions involving the IVC filters is to hire a lawyer who is handling these cases. That attorney will...
When will Bard IVC filters be refunded?
The litigation was closed to new cases and anyone who filed a lawsuit to be part of the litigation after May 31, 2019, would be given a full refund of the filing fee.
What is the IVC filter lawsuit?
Lawsuits claim that IVC filter manufacturers designed, manufactured and marketed defective devices. They also claim that the companies didn’t do enough to warn doctors and patients about the risks of the devices. Sherr-Una Booker had a Bard G2 filter implanted in 2007.
What was the warning letter to Bard?
Food and Drug Administration issued a warning letter to Bard in 2015 accusing two of the company’s plants of misfiling customer complaints and failing to notify the agency of serious malfunctions with its devices. The complaints included one of a patient death. The letter also accused the company of making and selling IVC filter retrieval devices the FDA had not cleared or approved.
How often are IVC filters used?
The study found that IVC filters were used 13 times as frequently in the U.S. as in five large European countries.
How long does it take for an IVC filter to be removed?
The FDA recommends that retrievable IVC filters should be removed between 29 and 54 days after they are implanted. The agency found that, after that amount of time, the devices pose a greater risk than the pulmonary embolisms they are supposed to prevent.
What companies are involved in IVC lawsuits?
Lawsuits name IVC filter manufacturers including Bard, Cook Medical, Rex Medical, and Cordis. Many claim that removing failed devices have been difficult to nearly impossible. Some claim continued mental stress because patients are forced to continue living with defective devices that it may be too risky to remove.
How much did Tonya Brand get in the Cook trial?
FEBRUARY 2019. A jury in the third Cook trial awarded Tonya Brand $3 million for injuries she received from her IVC filter.
What is an IVC filter?
IVC filters are small devices that are implanted into the inferior vena cava to “catch” blood clots that may break free inside the deep veins of the body and travel toward the lungs. The spider-like filters contain several legs, or struts, that expand inside the vein to prevent a pulmonary embolism.
How long does it take for a Tulip IVC filter to perforate?
According to the lawsuits, an April 2012 study published in the medical journal Cardiovascular Interventional Radiology found that 100% of Cook Celect and Gunther Tulip IVC filters perforated patients’ venal caval wall within 71 days of being implanted. The study also found that 40% of the filters became tilted and out of position.
When should you remove a filter?
The FDA recommended that doctors remove the filters once the danger of the clot has passed, to reduce the risk of the filters breaking free and traveling through the body .
What is the Injectafer lawsuit?
An Injectafer lawsuit indicates repeated iron infusion injections left a New York woman debilitated and with serious injuries.
What does this mean for my potential Bard IVC case?
Though no new cases may be filed into MDL 2641, you have two options: 1) New cases must be settled with Bard prior to filing a lawsuit; or 2) new cases must be brought individually in your home jurisdiction. It just cannot be filed as part of the MDL 2641 litigation.
Who is suing the manufacturers of IVC filters?
The Maher Law Firm , on behalf of our clients, is suing the manufacturers of certain IVC filters, including C. R. Bard Inc., and Cook. These IVC filters were supposed to catch any potential blood clots before the potentially deadly clots got into the heart and lungs. Instead, the filters are breaking, sending metal prongs throughout the body; they’re permanently lodging themselves in a patient’s IVC; they’re migrating to other vital organs, and; they’re even causing death.
How many cases are in the Bard MDL?
There are currently 8,443 cases pending in the Bard MDL. Judge Campbell is beginning the process of closing the MDL, and, as of May 31, 2019, no new cases may be filed into MDL 2641. On July 10, 2019 a report will be filed with the Court identifying the status of all cases in the MDL.
What is an inferior cava filter?
For people living every day with a higher-than-normal risk of blood clots, Inferior Vena Cava filters, or IVC filters, seemed like a means to an end to their health risks— and their fears. Only to find, after undergoing major surgery, these IVC filters have failed them and created more problems than they originally had.
When is the deadline for a case categorizing order?
The deadline to comply with the Case Categorization Order was February 7, 2019. As a result of the Court’s Order, a significant number of cases were dismissed because the plaintiff was unable to substantiate an injury or failed to correctly submit their case categorization form.
Is there a settlement in the Cook v. Cook case?
There have no major public settlements in the Cook litigation and Cook has taken the position that it is unwilling to settle cases at this point in the litigation.
Is MDL 2570 still pending?
MDL 2570 against Cook and its related entities is still pending. Judge Young of the United States District Court for the Southern District of Indiana oversees all Cook IVC filter litigation. As of June 19, 2019 there are 6,343 cases pending against Cook.
What is Bard IVC filter?
Bard has manufactured many types of inferior vena cava (IVC) filters that are currently the subject of recalls and mass tort litigation for alleged defects. If you have been injured by a Bard IVC filter, you may be eligible to recover damages through an IVC filter lawsuit. 1. General information. 2.
What is the design defect of Bard's inferior vena cava filters?
The central alleged design defect of Bard’s inferior vena cava filters is that some may fracture and/or migrate throughout the patient’s body. Not only does compromise the IVC filter’s efficacy in managing blood clots. The broken pieces can also perforate and lodge into veins and other organs, causing serious injury and possibly death.
How many Bard MDL bellwether trials have been concluded?
So far three Bard MDL bellwether trials have concluded. Two did not find Bard liable for the plaintiffs’ injuries:
How much did Bard pay to a victim?
Bard was recently ordered to pay $3.6 million to a victim injured by an IVC filter.
Why was Bard's Denali filter recalled?
The FDA temporarily recalled Bard’s Denali filter in 2015 for labeling problems. That same year, the FDA issued a warning letter that claimed Bard:
What will happen after the bellwether trial?
Once the final bellwether trial is concluded, both sides will probably negotiate for a global settlement to settle all the cases.
Where is Bard located?
Currently Bard is headquartered in Murray Hill, New Jersey and employs about 14,000 people. The general phone number is (201) 847-6800.
How many deaths were caused by Bard IVC filters?
In 2015, NBC News linked Bard IVC filters to 39 deaths. The network’s investigation claimed that Bard executives had been aware of the risk for years but the company did nothing.
What is the IVC filter lawsuit?
IVC filter lawsuits claim C.R. Bard and Cook Medical’s devices were defective, making them more likely to fracture or perforate the inferior vena cava. In March 2018, Bard was ordered to pay a woman $3.6 million to settle an IVC filter case. Bard and Cook have agreed to individual IVC filter lawsuit settlements for undisclosed amounts.
How much did the Bard Bellwether verdict cost?
The first Bard bellwether trial resulted in a $3.6 million verdict for a Georgia woman. The jury awarded $1.6 million in actual damages and $2 million in punitive damages to Sherr-Una Booker.
What happens when IVC filters break?
In some cases, when the devices break, pieces can travel through the body, damaging the heart, lungs and other organs.
How many lawsuits have been filed against IVC filter makers?
As of July 2019, more than 14,000 lawsuits have been filed against two IVC filter makers. Cook Medical faced 5,627 lawsuits in an Indiana federal court. Bard faced another 8,423 in an Arizona federal court.
Why do people use IVC filters?
IVC filters are designed to reduce the risk of blood clots in people who are unable to take blood thinners. But people claim IVC filters punctured their veins, broke or moved, leading to serious IVC filter complications.
When was the Bard trial?
It was set for April 30, 2018, but the judge said the plaintiff waited too long after his injury to file suit. March 30, 2018. A jury in the first Bard trial awarded a $3.6 million verdict to Sherr-Una Booker. She claimed a Bard IVC filter broke and injured her.
